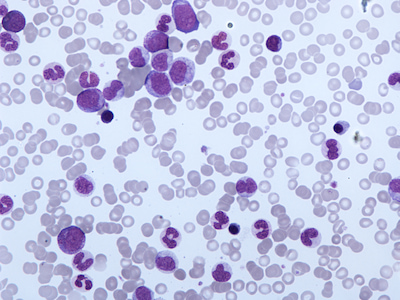
Optimal Care Pathways for myelodysplastic syndromes (MDS) patients
- Myelodysplastic syndromes
- Myeloproliferative neoplasms



In May 2020, Fiona was diagnosed with Myelodysplastic Syndrome. Also known as MDS, it is a type of blood cancer that affects the production of normal blood cells in the bone marrow. Fiona shares what it was like to be diagnosed with a rare blood disease as a mother of five, wife and nurse, in the midst of the COVID-19 pandemic.

A Phase I investigator-driven clinical trial, which is ready to recruit its first participant, gives Australian patients with high-risk MDS access to two new oral drugs.

Don Hayes had an allogeneic stem cell transplant days before his 71st birthday.


Tony Wakely’s diagnosis with MDS quickly transformed to AML, which he beat with a transplant. But a year later, he was shocked to find out his MDS had come back.

Two new international clinical trials are expected to improve access to new therapies for Australians with MDS as part of the Leukaemia Foundation’s Trials Enabling Program (TEP).

The Leukaemia Foundation provided consumer comments to the Pharmaceutical Benefits Advisory Committee (PBAC) in relation to Otsuka Australia Pharmaceutical’s resubmission for decitabine and cedazuridine (Inqovi®) for high-risk MDS and CMML.